Lipids are important macromolecules within our body. When people hear the word “lipid”, they typically think of fats. However, lipids are actually a diverse group of compounds, which include oils, hormones and fats.

Glycerol is typically the backbone for complex lipids, such as glycerides. Glycerol is colourless, odourless and typically has a sweet taste to it. It is a three-carbon alcohol and a major “acceptor” for fatty acids.
It is important to understand that fatty acids are typically unreactive molecules. They are happy to do their own thing, lounge around and have a good time. Our bodies don’t necessarily like this though – fatty acids can be quite useful. So, we add a molecule named coenzyme A to make fatty acids a bit more… snappy.
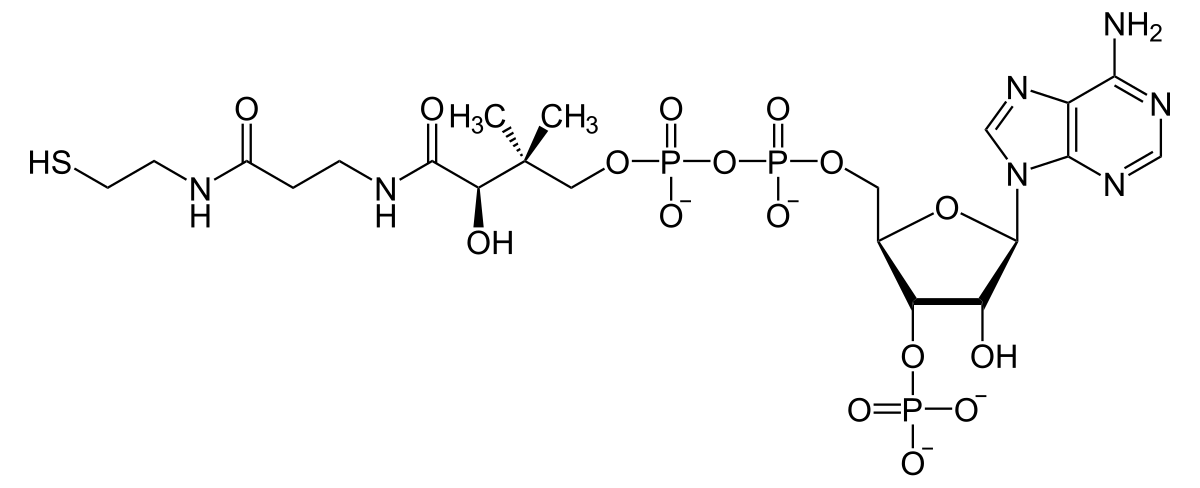
Coenzyme A contains a highly reactive thiol group named acyl CoA.
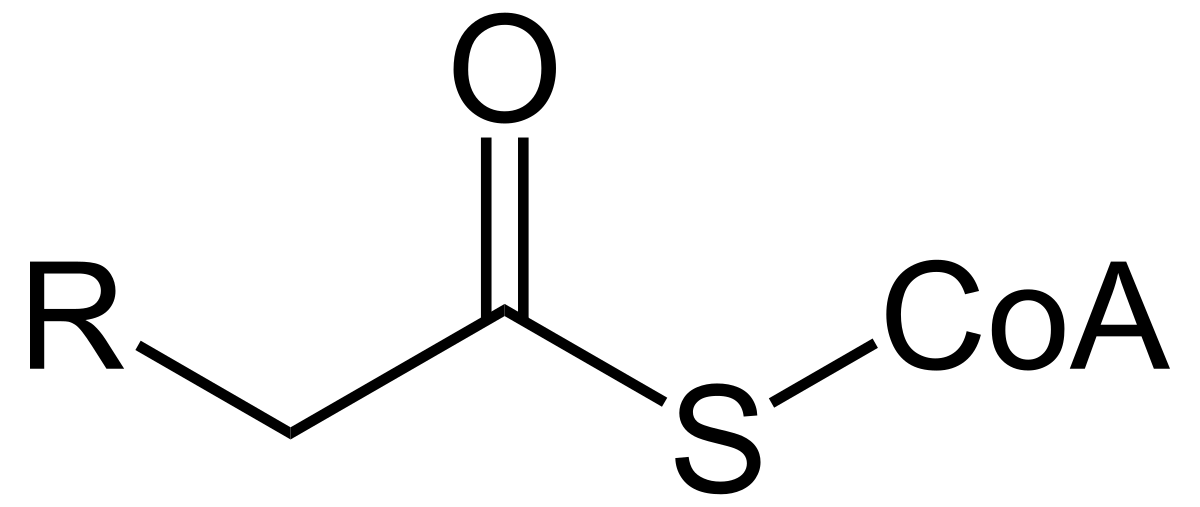
When we use coenzyme A, acyl CoA is added to the carboxyl group of fatty acids. This enables fatty acyl CoA to react with the alcohols on glycerol to generate di and triglycerides.
Di and triglycerides are both particularly important molecules. However, today we are going to focus on diglycerides.

Diglycerides are particularly important for the synthesis of phospholipids and acting as signalling molecules.
A phospholipid is a lipid made of glycerol, two fatty acid tails, and a phosphate-linked head group. These head groups can be: serine (-), inositol (-), or neutral with both positively and negatively charged coups: cholin, ethanolamine.
All phospholipids are amphipathic. This means they have both hydrophilic (water-loving) and hydrophobic (water-hating) parts. In this molecule, the hydrophilic component is the polar end – aka the phosphate and ‘x’ head group. The negative charge of the phosphate makes it water loving. The hydrophobic component is the non-polar end – aka the two fatty acid tails. A way to remember this is to imagine oil in water – they do not mix, no matter what you try. You can shake it, heat it, freeze it, but they will not mix. Fatty acids will not mix with water.
It is important to note:
- Saturated fatty acids are localised to C-1.
- Unsaturated fatty acids are localised to C-2.
- The ‘X’ site (head group) attach at C-3.
Phospholipid structure is classified according to the head group and acyl chain composition. Thus, they are named phosphatidylserine (PS), phosphatidylcholine (PC), phosphatidylethanolamine (PE), phosphatidylinositol (PI).

Another important lipid are the sphingolipids. The backbone of sphingolipids is sphingosine, which is analogous to the glycerol moiety in phospholipids.
It is important to note:
- A fatty acid chain is attached via amide linkage to the NH2 molecule on C-2.
- The polar head group is attached at C-1.
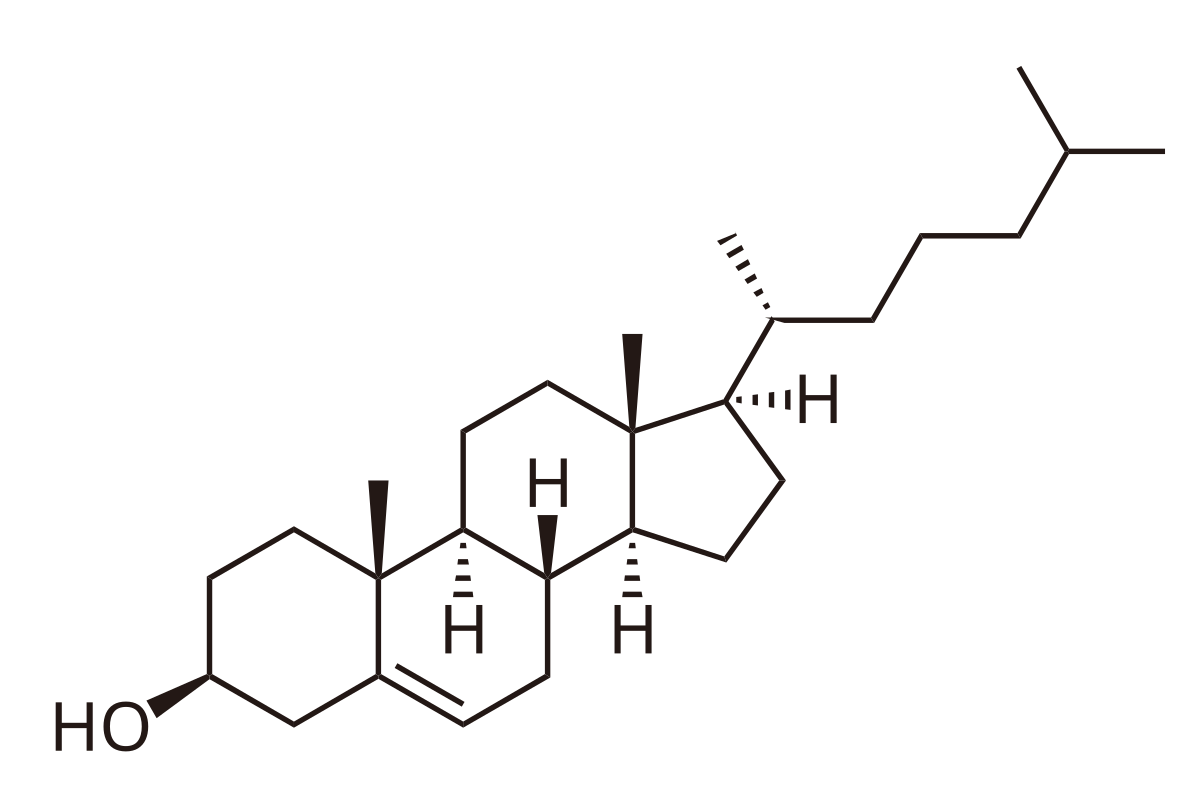
Yet another important lipid is cholesterol.
Cholesterol is a 27 carbon compound built from units of acetyl-CoA (not to be confused with acyl CoA!).
Cholesterol is a highly rigid, hydrophobic molecule. Its ampiphillicity arises from the hydroxyl moeity.
Each lipid structure within our bodies are determined by the physical and chemical properties of their constituents. Micelles and liposomes form spontaneously in aqueous solutions. Cholesterol intercalates into either structure.